The transition from high Global Warming Potential (GWP) propellants such as HFA134a to low-GWP alternatives such as HFA152a and HFO1234ze(E) in pressurised metered dose inhalers (pMDIs) poses challenges for inhaled pharmaceutical product development.
Changes in the chemicophysical properties of products formulated with low-GWP propellants such as density and viscosity will alter product performance, impacting in-vitro bioequivalence metrics. This study investigates those differences using equivalent pMDI hardware and formulations.
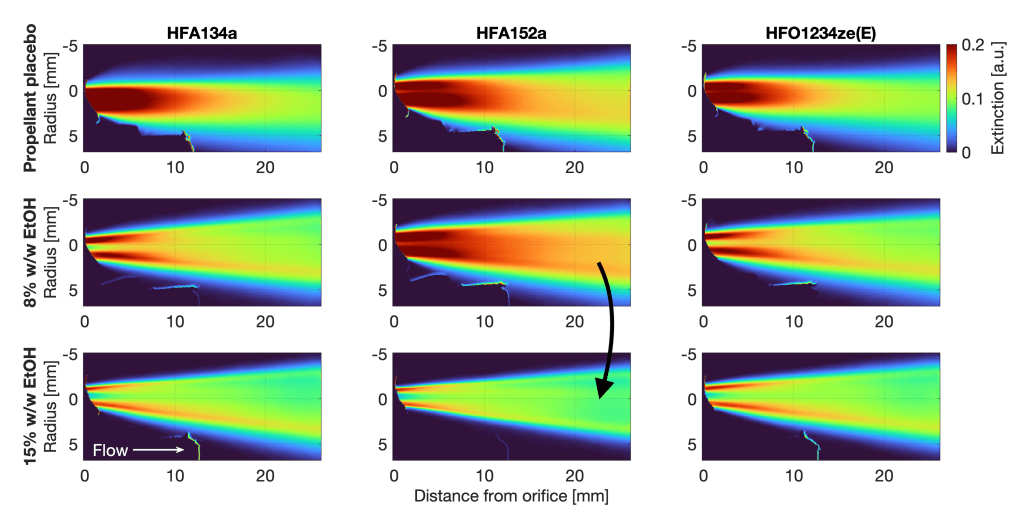
In this paper, we’ve compiled the largest-yet database of high-resolution plume geometry and aerodynamic particle size distributions for a generic becomethasone dipropionate solution formulation with various co-solvent concentrations. With a sufficiently large, high-resolution dataset, small variations in the repeatability and stability of the sprays produced by different propellants become evident, and this helps explain why deposition and drug delivery varies.

You must be logged in to post a comment.